Gut Microbes, Gut Feelings and Our Brains

The gut and the brain constantly talk to each other- and it's a two-way conversation! This communication is assisted by nerves and by blood (Figure 1).
An important player in this cross-talk is the vagus nerve. This nerve runs from the brain, down to the gut and other organs like the heart, lungs and liver. For the purpose of this blog, we'll focus on the direct connection between the brain and intestines - but it's important to remember that all these organs are connected in a complex network.
Skip to content:
- The microscopic view of the gut
- “All disease begins in the gut”
- Valuable lessons from animal models
- The vicious stress cycle and the hope for an escape
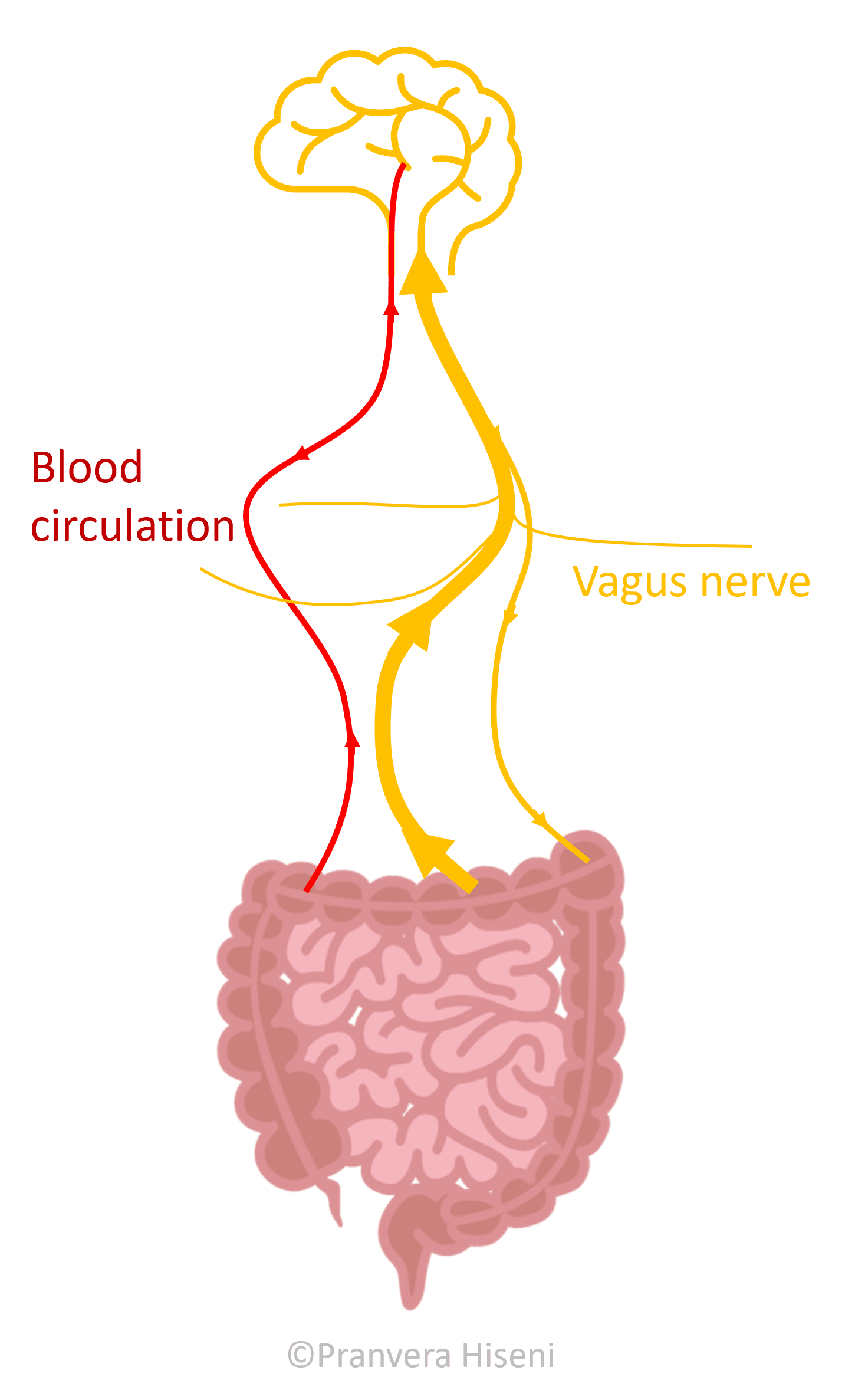
The vagus nerve is mostly made of sensory fibres; fibres that collect impulses from the gut and send them to the brain. These become excited any time the intestinal muscles stretch or experience any type of mechanical pressure, in addition to sensing different types of molecules that are produced either by the gut lining or gut microbiota (i.e., gut microbes).
The impulses gathered by this nerve are directed towards different parts of the brain. For example, vagus communicates with amygdala, which is the centre for emotional behaviour, motivation and fear. It also sends signals to prefrontal cortex, a complex part of our brains responsible for high level cognition, decision making and social behaviour.
Vagus also has motoric fibres which send signals from the brain back to the gastrointestinal muscles. Motor fibres regulate muscle contraction and secure an intact intestinal barrier. It is described that many people suffering from chronic constipation, wherein the movement of bowels is slow, have a dysregulated stimulation of the intestinal muscles by this nerve.
The other line of interaction between the gut and the brain involves gut metabolites travelling to the brain via blood circulation. This is possible since many of the molecules originating from the gut pass the intestinal and blood brain barriers. In addition, many hormones produced in the brain reach the gastrointestinal tract through blood.
The microscopic view of the gut
To further understand the gut-brain axis, it is important to also focus on the major components of the gut itself. There is gut epithelium, a barrier that separates the intestinal lumen where trillions of microorganism cells live from lamina propria, where the nerves, capillaries and immune cells reside (Figure 2). The epithelium cells are tightly connected to one-another to ensure an intact barrier, so that no macromolecules (for example food particles) can pass freely from one side to another. As long as the connections between cells are functional, only small molecules can naturally pass through them.
On the other hand, there are cases when the connections between cells are compromised, which means that the control over what passes across the barrier is lost. This is known to be the case for many functional gastrointestinal diseases, but not only. A leaky gut which allows the passage of different macromolecules across barriers has also been linked to neurodegenerative diseases, such as Parkinson’s and Alzheimer’s disease, but also depression.

Let us again bring the microorganisms into the picture. They constantly digest the food that we eat. While they do so, they produce various types of small molecules which are allowed to pass from lumen to lamina propria. Some of them are short chain fatty-acids (SCFAs), like propionate, butyrate or acetate, some are serotonin (the “happy hormone”) precursors, and some other molecules.
Different microbes are specialized to produce different beneficiary molecules, so it is important that there is a diversity of them in our guts. For example, it is very important that among others, the gut is colonized with butyrate-producers. Butyrate is a SCFA that serves as an energy source to gut epithelial cells, helping maintain its integrity. A gut low in butyrate-producers has increasingly been linked with various health conditions, including colorectal cancer and obesity-related metabolic diseases.
On the other hand, there is another SCFA, called isovaleric acid, mostly produced by two bacteria groups, Alistipes and Oscillobacter. This acid is known to be neurotoxic; it passes gastrointestinal barrier and is transported to the brain through blood circulation, where it exercises its neurotoxicity. High levels of these bacteria and isovaleric acid have been found in the stool of people suffering from depression.
“All disease begins in the gut”
It has long been observed that people suffering from Parkínson’s Disease also suffered from gastrointestinal issues - such as severe constipation - for years prior to the manifestation of disease itself. It is now being widely accepted that the disease may have its roots in the gut. And not only Parkinson’s, but Alzheimer’s, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) as well.
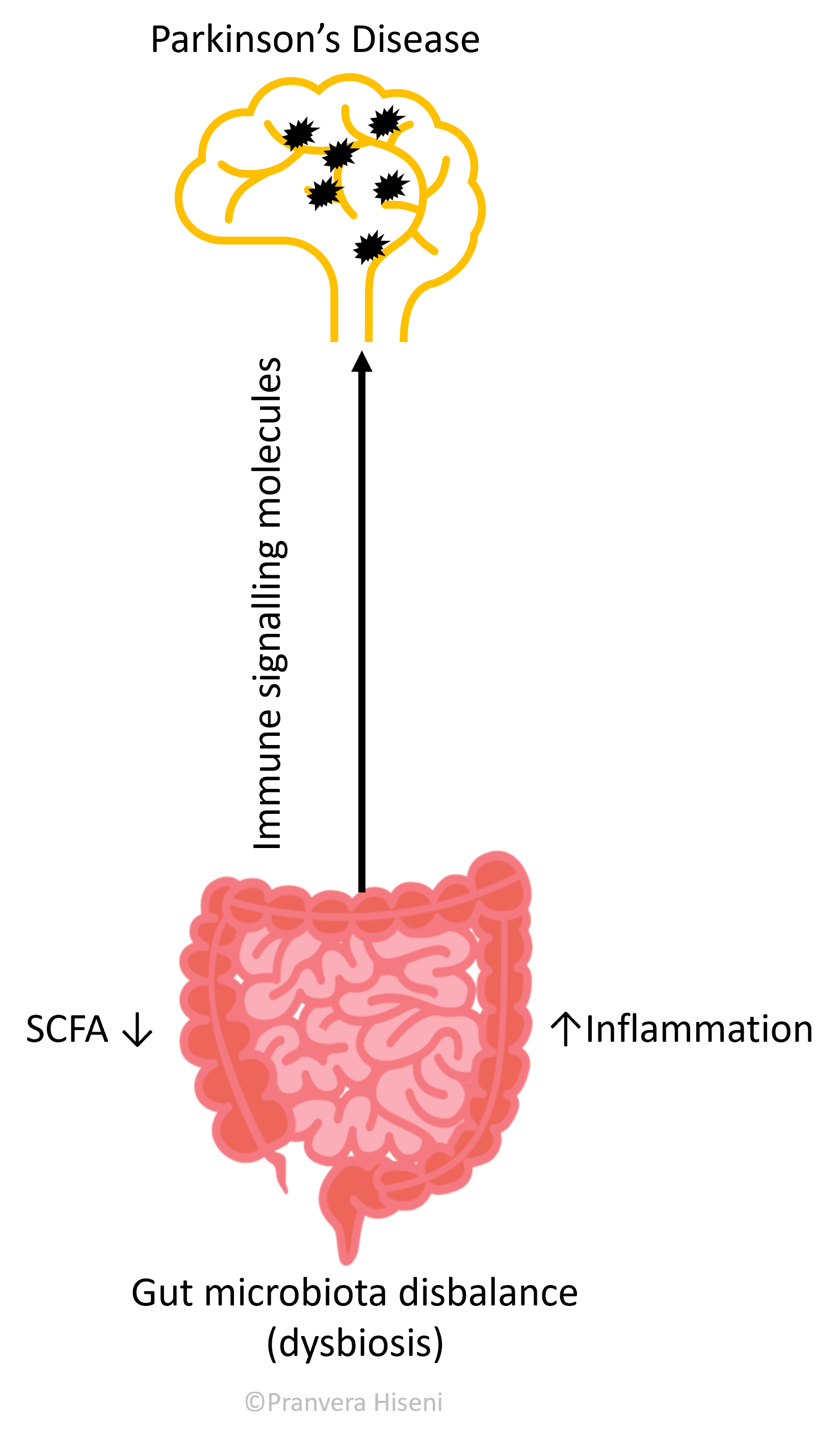
It has been shown that people suffering from these diseases have a different microbiota composition as compared to healthy controls, a state described as dysbiosis.
One potential route of disease pathogenesis in the case of Parkinson’s is a triggered gut inflammation, resulting in the local production of various immune system signalling molecules. These molecules are then transported to the brain via blood, promoting an overproduction of a protein called alfa-synuclein in nerve cells. The production rate of these proteins results faster than their destruction by the nerve cell machinery, so they form aggregates which in turn make the brain cells dysfunctional (Figure 3).
The GA-map® Dysbiosis test captures main butyrate-producers like Faecalibacterium prausnitzii or Eubacterium rectale, in addition to detecting Alistipes. For each incoming sample, the abundance of these bacteria is compared to the one found in a healthy reference population. The results are presented in the patient report form accompanying the calculated Dysbiosis Index score.
Valuable lessons from animal models
One way of exploring the effect of gut microbiota on the brain and behaviour is by studying germ-free animals, usually mice. Such mice are brought into the world in sterile conditions. They live in germ-free chambers throughout all their life. The basic purpose of studies in these animals is to analyse their overall development and behaviour and compare it to genetically identical mice that are raised in a conventional manner and therefore colonized by microorganisms.
It has been shown that in germ-free animals, the blood brain barrier is not fully formed. This barrier controls the passage of molecules from blood to the brain, preserving the brain from direct contact with various molecules circulating in the blood system. When the barrier is not fully formed, the brain remains exposed to all sorts of molecules, such as glucocorticoids for example, which negatively affect the neurogenesis during early development.
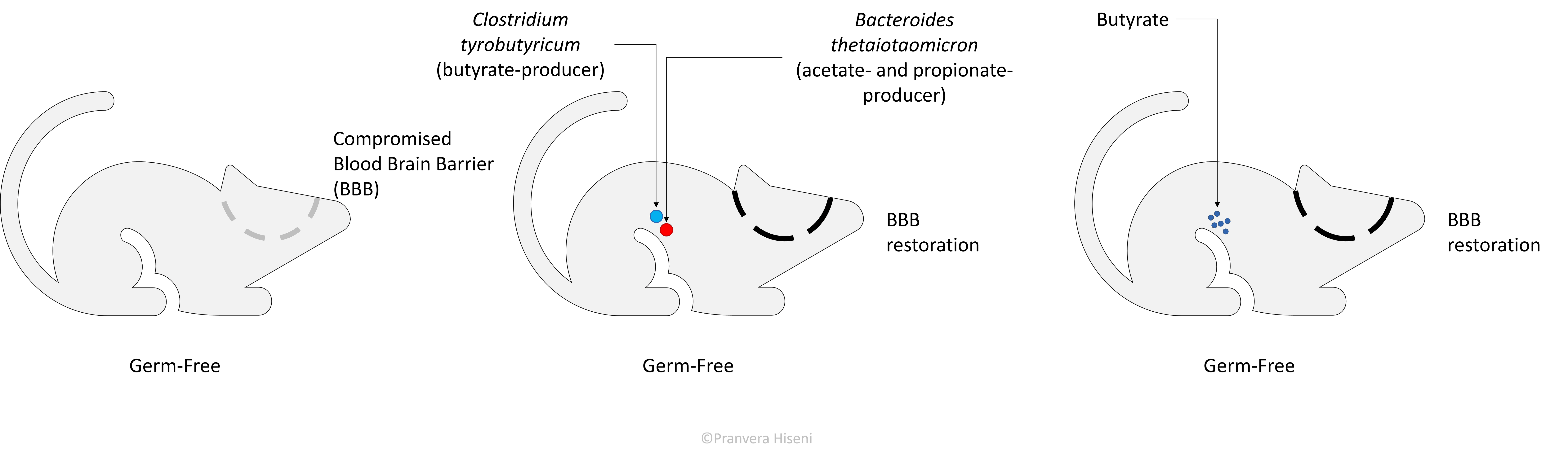
A hallmark study performed by Braniste et al. on 2014, showed that gut microorganisms may regulate this barrier by modulating the protein expression of its tight junctions (i.e., regulate the cell connections in the blood-brain barrier structure).
This is thought to be mediated through the production of three main short chain fatty acids, acetate, propionate and butyrate. Germ-free mice treated either with Clostridium tyrobutyricum, a known butyrate-producer, or Bacteroides thetaiotaomicron, a propionate- and acetate-producer, showed an improvement in the integrity of blood brain barrier. Same effects were observed when germ-free mice were directly treated with butyrate, linking the effect of microbial metabolites to an improved blood brain barrier integrity (illustrated on Figure 4).
It is important to note that a compromised blood brain barrier and a disbalanced gut microbial community has been found in children diagnosed with autism spectrum disorders. Knowing that the blood brain barrier is formed in early infancy, starting from intrauterine life, these types of results show the importance of gut microbial composition in both mothers and babies for a normal brain development.
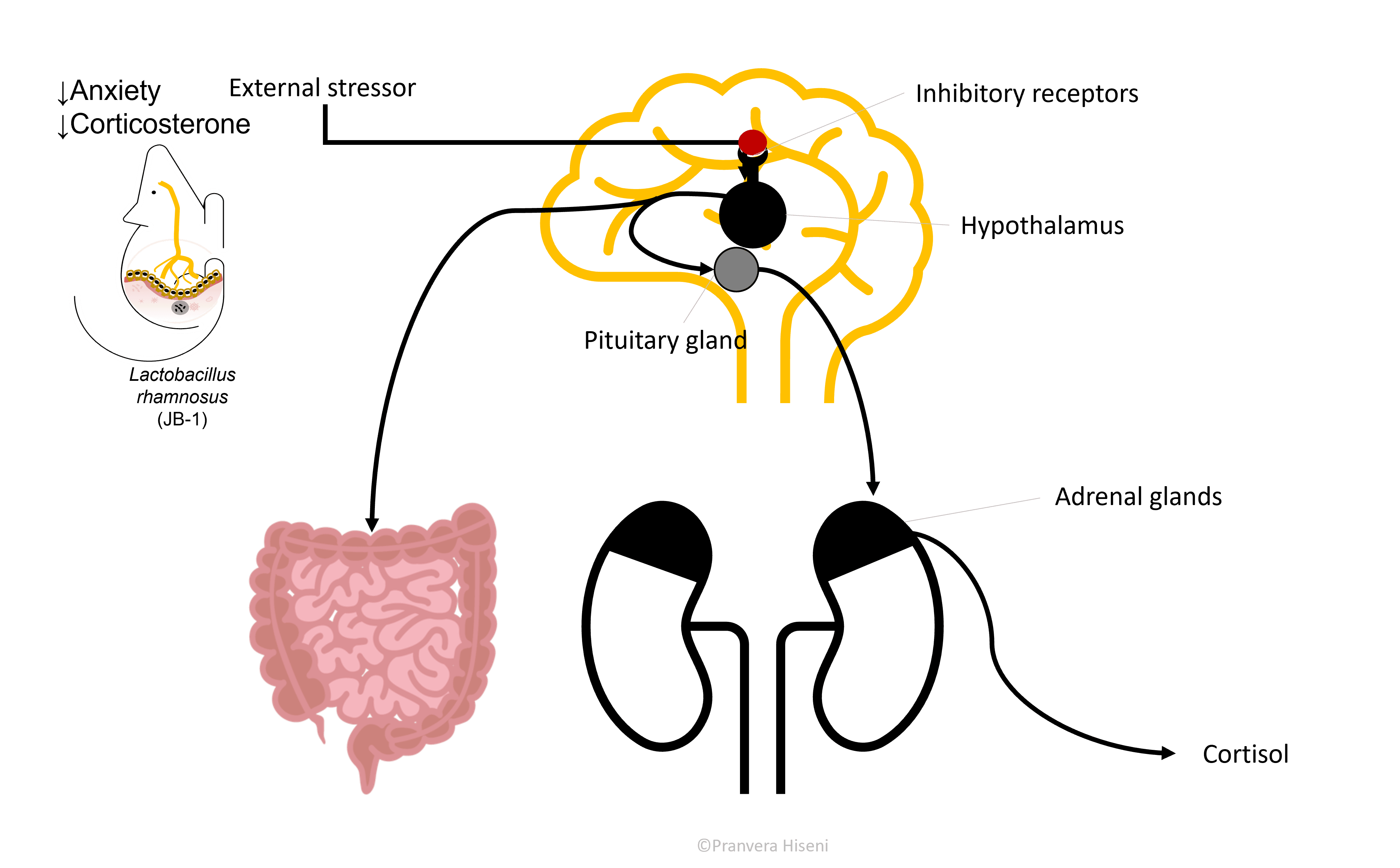
Let us bring back the vagus nerve and intestinal microorganisms into the picture. An important study performed by Bravo et al., has shown that healthy mice treated with Lactobacillus rhamnosus - a known probiotic, exhibited reduced anxiety levels, had improved emotional behaviour and lower corticosterone levels in their blood.
Corticosterone is a marker of stress response, similar to cortisol in humans. Authors proved that the effect of the bacterium was exercised through the vagus nerve, because no beneficiary change was observed in mice where vagus was removed. This is a very important finding because it gave a definitive proof that vagus nerve is directly stimulated from bacteria, and that this in turn affects the host brain. They observed that mice treated with Lactobacillus rhamnosus had an increase in inhibitory receptors in hypothalamus, only when vagus was active. Why should a regulated inhibition in the hypothalamus be important? We will find out more in the following sections.
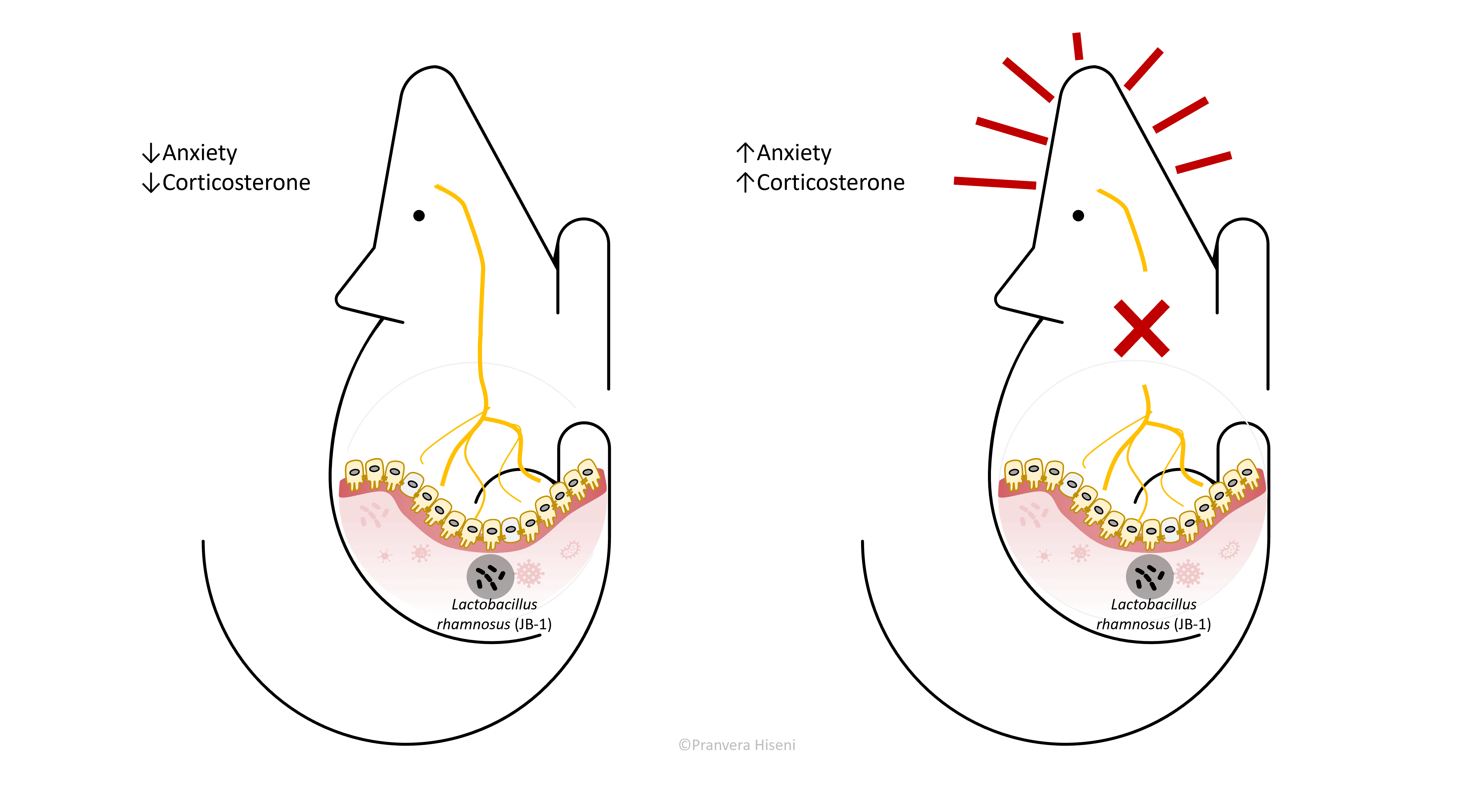
Anxious mice are more scared to be exposed to open and brightly lit fields. They spend most of the time nearby walls in the darkest areas of the chamber.
The vicious stress cycle and the hope for an escape
Hypothalamus, together with Pituitary and Adrenal gland form an axis (HPA axis) that is responsible for stress hormone production, cortisol in us or corticosterone in rodents. This is an important and a beneficial hormone in times of fight-or-flight situations. It helps the brain focus and use the available blood sugar by shutting down any unessential function that would otherwise be harmful in emergency situations.
But often in individuals suffering from depression and anxiety, cortisol production is ongoing, even in times with no actual stressors stimulating its production. So, it is logical to expect that an inhibition, or better said a regulation in hypothalamus, would result in reduced cortisol level production (Figure 6).
Let us now form a full circle and come back to the gut. When hypothalamus is stimulated, it produces a hormone called corticotropin-releasing hormone, which further stimulates the pituitary gland on the way of producing cortisol. But this hormone also travels to the gastrointestinal tract and exercises an effect there. The effect of this is marked by an increased intestinal permeability because this hormone loosens up the junctions between the gut epithelial cells.
This in turn induces changes in the gut microbiota composition which is now in a more direct fight with the immune system. This is thought to be the case in many patients suffering from irritable bowel syndrome and inflammatory bowels disease, both highly correlated with anxiety and depression.
We must perform another half-circle here. Microbes in the gut are known to stimulate the communication between immune cells. In case of a leaky gut, the immune cells produce pro-inflammatory signal molecules, called cytokines, which travel to the brain and stimulate the hypothalamus, bringing us once again to an increased stress response.
It is time for a short recap here: if the body is under external pressure, it will elevate the stress hormone levels which in turn can cause the gut to leak. This will then change the microbiota composition, reducing the diversity by killing beneficial bacteria. A leaky gut will put macromolecules in a direct contact with immune cells, who will produce signalling molecules that add to the amount of stress for the hypothalamus to process (Figure 7).
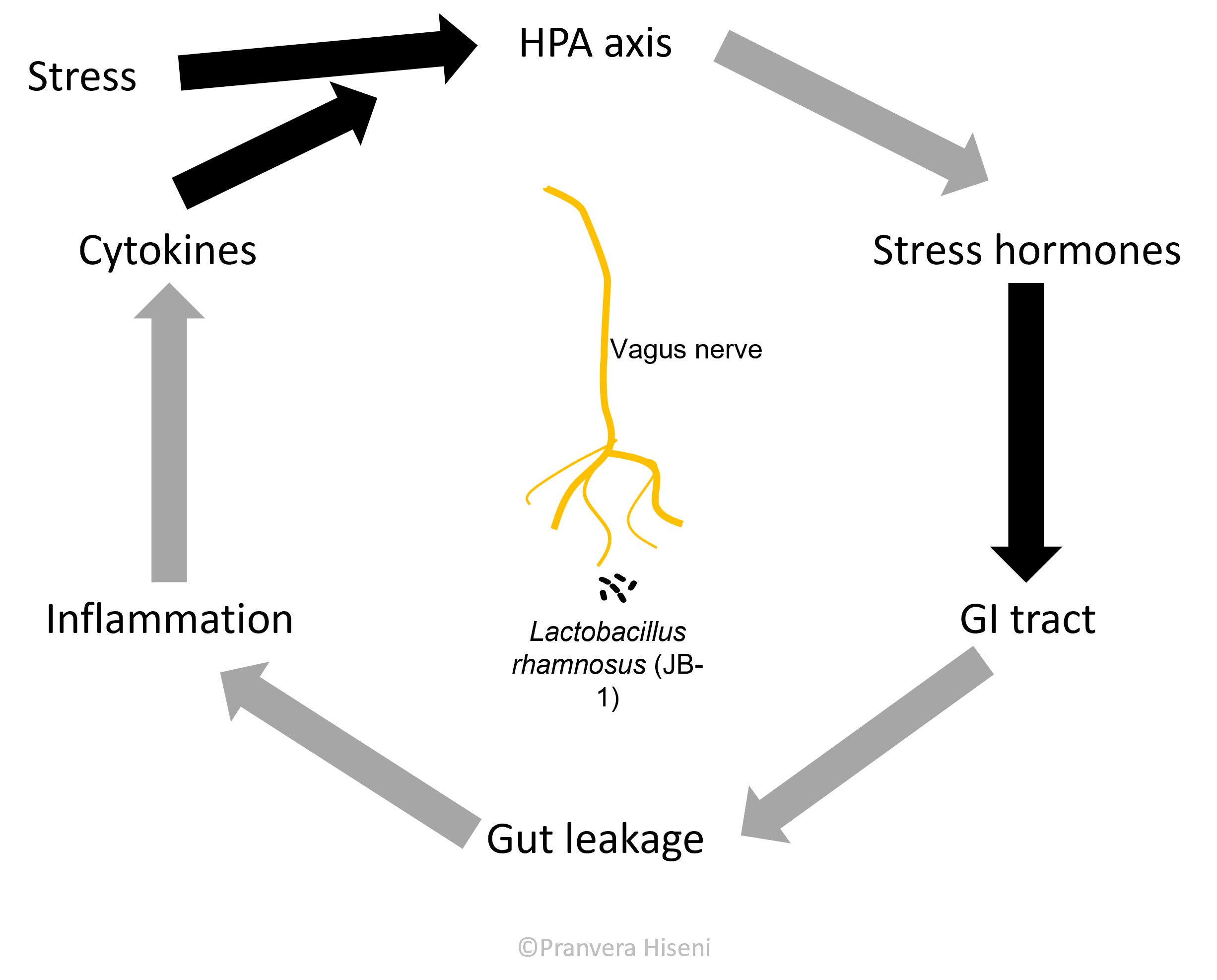
However, we now have proof that beneficial gut bacteria can regulate the stress response axis by inhibiting its activity in the brain. So, we might be close to finding a way of stopping this spiral of events that keep making the situation worse.
It might very well be that minor lifestyle changes - like regular sleeping hours, regular consumption of fermented foods like yoghurt, kimchi, sauerkraut, more fiber in our diet, limited antibiotic exposure – will have a major effect on our overall wellbeing. On this note, a regular monitoring of gut dysbiosis is a great way of identifying whether the “lions” that keep chasing us are simply an echo of an internal disbalance.
