Know your microbes: Escherichia and Shigella

Key players in the human gut: Escherichia and Shigella
The human gut is home to millions of bacteria – the gut microbiome, a community of microbes contributing to our digestion, metabolism, and immune defense. As our knowledge of the microbiome advances, roles of some bacteria in the human gut become clearer. Escherichia and Shigella, although commonly connected to disease outbreaks, are not exclusively pathogens. Here is what science has taught us so far.
Escherichia and Shigella – key players in gut health and disease
Most people, regardless of their scientific knowledge have heard of E. coli, or Escherichia coli, a bacterium making regular media appearances when causing foodborne disease outbreaks. However, these outbreaks only happen when specific types of E. coli, such as EIEC (enteroinvasive E. coli), are involved. Closely related to Escherichia, yet slightly less known to the western public, Shigella is a disease-causing bacterium. Shigella species stand behind the majority of dysentery outbreaks among children in the developing countries.
However, certain types of Escherichia/Shigella are fast residents of the human gut and sometimes they can even prove beneficial.
The Role of Gut Microbiota in Human Health: Benefits of Anaerobic Bacteria and SCFA Production
Within the healthy human gut there is a dense bacterial community (i.e., gut microbiota) of mostly anaerobic bacteria that contributes to human health through various functions including protection against colonization of pathogenic bacteria and immune modulating effects. For instance, by breaking down complex carbohydrates (such as fiber), colonic bacteria produce metabolites called short-chain fatty acids (SCFAs) that have several beneficial effects, such as regulating the immune system and metabolic pathways, and maintaining the gut barrier function and the anaerobic gut environment.
Escherichia and Shigella: Understanding Their Role in the Gut Microbiota
Escherichia and Shigella are part of the Enterobacteriaceae family of bacteria in the diverse Pseudomonadota phylum (previously called the Proteobacteria phylum). Pseudomonadota, including Escherichia/Shigella, are a normal part of the adult gut community, however, are usually found in low abundance. The Enterobacteriaceae family are the most abundant Pseudomonadota in the gut – of which E. coli is the most common.
Pseudomonadota are facultative anaerobic bacteria, which means they can survive both in the presence and absence of oxygen. Bacteria from the Enterobacteriaceae family are among the first colonizers of the oxygen-containing gut in newborns. However, a quick reduction of oxygen favours growth and dominance by strict anaerobic bacteria – which persist into adulthood.
Separating Escherichia and Shigella
Escherichia and Shigella are often talked about together, and for a long time, Shigella spp. were considered a part of E. coli species. This is not entirely wrong, as EscherichiaEscherichia are genetically closely related to Shigella. The two are difficult to separate using a gene commonly used to detect and identify bacteria (the bacterial 16S rRNA gene). An accurate identification is especially crucial when infection with pathogenic E. coli or Shigella variants are suspected in clinical context. To provide accurate answers when identifying these disease-causing bacteria strains, diagnostic laboratories rely on biochemical testing and toxin detection.
Beneficial and Pathogenic Variants of E. coli: A Probiotic and Pathogen Perspective
There are both beneficial and harmful variants of E. coli bacteria. For instance, a probiotic Escherichia coli strain, originally isolated from the stool of a soldier that remained unaffected during an outbreak of Shigella (E. coli Nissle 1917), has been used to treat e.g., infectious diarrhoea and inflammatory bowel disease. On the other hand, E. coli infections by pathogenic strains can cause diseases such as enteritis, diarrhea, and urinary tract infection.
Dysbiosis: How Escherichia and Shigella Become Problematic in an Imbalanced Gut
In a healthy gut, bacteria from the Enterobacteriaceae family, such as Escherichia typically exist in low abundance and play non-harmful roles. However, when dysbiosis occurs—an imbalance in gut microbiota composition—these bacteria can become problematic. Dysbiosis often results in an increase in facultative anaerobic bacteria like Escherichia and Shigella, which thrive in conditions where oxygen levels rise due to inflammation or antibiotic use. This shift disrupts the dominance of beneficial anaerobic bacteria and compromises the gut barrier, leading to conditions like inflammatory bowel disease (IBD) or irritable bowel syndrome (IBS).
From Gut Dysbiosis to Systemic Diseases
Given the importance of the gut microbiota for human health, disruptions of the gut bacterial composition and dysbiosis are associated with numerous diseases. An increase of Pseudomonadota has frequently been observed. For instance, Pseudomonadota-rich dysbiosis is associated with more severe or aggressive inflammatory bowel disease (IBD), and some strains of invasive E. coli have been recovered from the inflamed tissue of Crohn’s disease patients. Elevated Pseudomonadota, including Escherichia/Shigella, have also been reported in other diseases, such as irritable bowel syndrome (IBS) and colorectal cancer.
Environmental Factors That Influence the Growth of Escherichia and Shigella
Growth of potentially harmful Enterobacteriaceae including E. coli, may be promoted due to e.g., inflammation or antibiotic treatment, which can increase oxygen levels and reduce the abundance of commensal anaerobic bacteria. As this may harm gut integrity, high abundance of Pseudomonadota or Enterobacteriaceae may indicate a compromised gut barrier. Enterobacteriaceae may also trigger or enhance inflammation, via e.g., immune-stimulating surface molecules.
Diet, Escherichia/Shigella and gut health
In the case of gut microbiome, what we eat truly matters. Diet is an important factor that may affect bacterial abundance and balance. Some food components also provide nutrition to the bacteria. Enterobacteriaceae, including E. coli, may use sugars such as glucose as a source of energy. Escherichia, like most bacteria, are also dependent on iron for growth. Multiple studies show that following certain dietary patterns can also modulate the gut microbiome. A high-fat diet is associated with increased abundance of Pseudomonadota, while a typical western diet has been associated with increased abundance of Escherichia and E. coli. Conversely, a diverse diet, with intake of complex carbohydrates, is associated with lower abundance of Enterobacteriaceae and Escherichia/Shigella and increase in beneficial bacteria.
Balanced microbiome - why the "big picture" matters
Examining individual bacterial species in isolation often does not provide a comprehensive picture of a person’s overall health. The gut microbiome is a highly complex and dynamic ecosystem, where intricate interactions between different microbial communities play a crucial role in maintaining balance and overall well-being. Assessing the gut microbiome holistically, rather than focusing on singular bacteria, has become an invaluable tool in modern medicine. Dysbiosis—an imbalance in the gut microbiota—can manifest in diverse ways, influencing digestion, immune function, metabolism, and even neurological health.
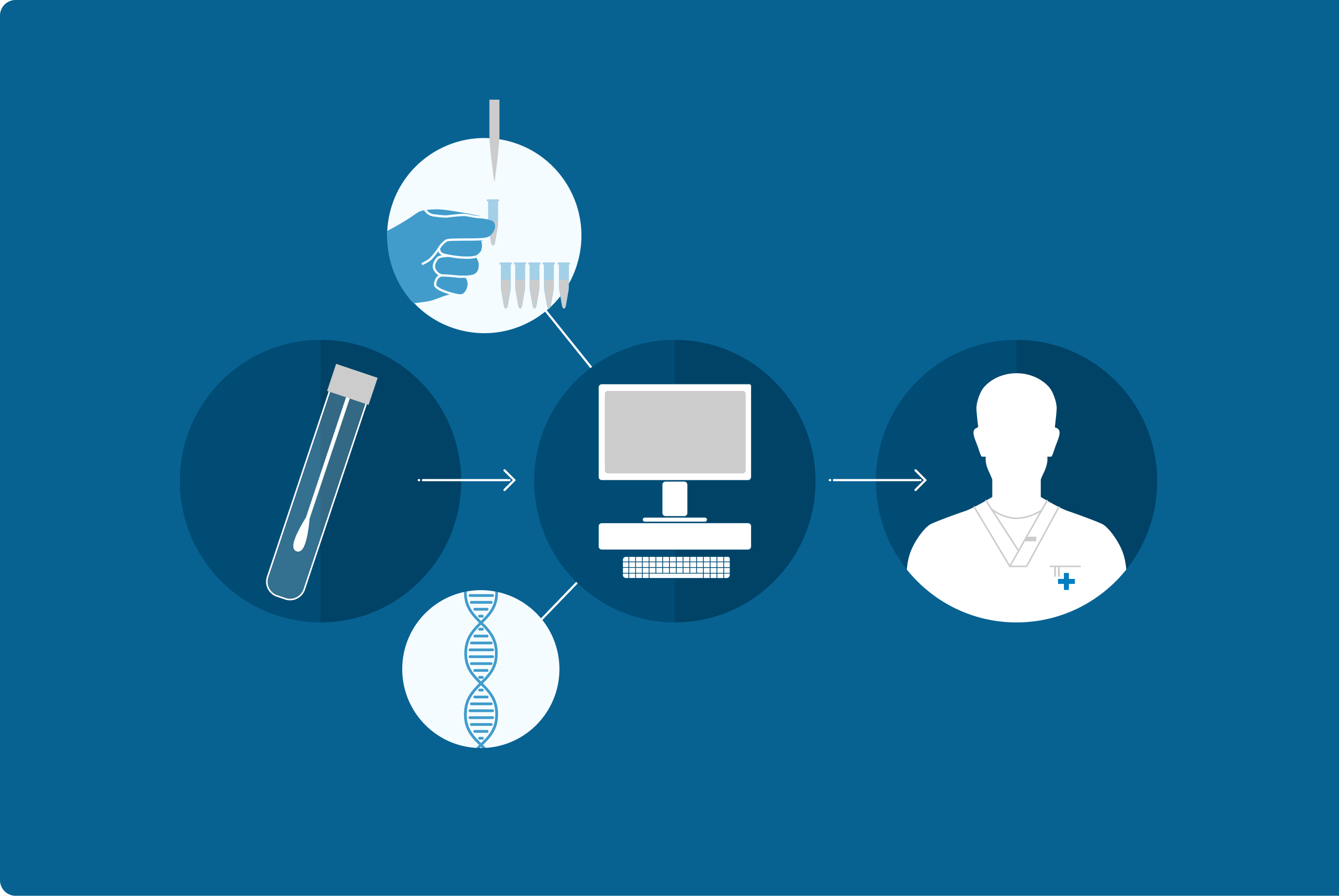
Growing evidence suggests that disruptions in the gut microbiome are not only associated with various symptoms but can also contribute to the development and progression of medical conditions. The GA-map® Dysbiosis Test offers laboratories and clinicians a standardized and innovative diagnostic tool for evaluating gut microbiome balance. By analyzing key bacterial profiles, the test enables healthcare professionals to identify dysbiosis, facilitating more personalized and effective treatment approaches for patients.
